It is one of the rarest and deadliest diseases in the world. GM1 Gangliosidosis. Worldwide, approximately 6,000 children suffer from this disease. Mila Kapturczak, from Mönchengladbach, Germany, also was diagnosed with the fatal disease at the tender age of just 5.
Please add the message “Mila” to your PayPal donation
You can also donate by bank transfer
Recipient: Löwenkinder Viersen
Purpose of use: MILA
Account: Sparkasse Krefeld
IBAN : DE65 3205 0000 0000 4708 80
You will receive a donation receipt
Mila Kapturczak
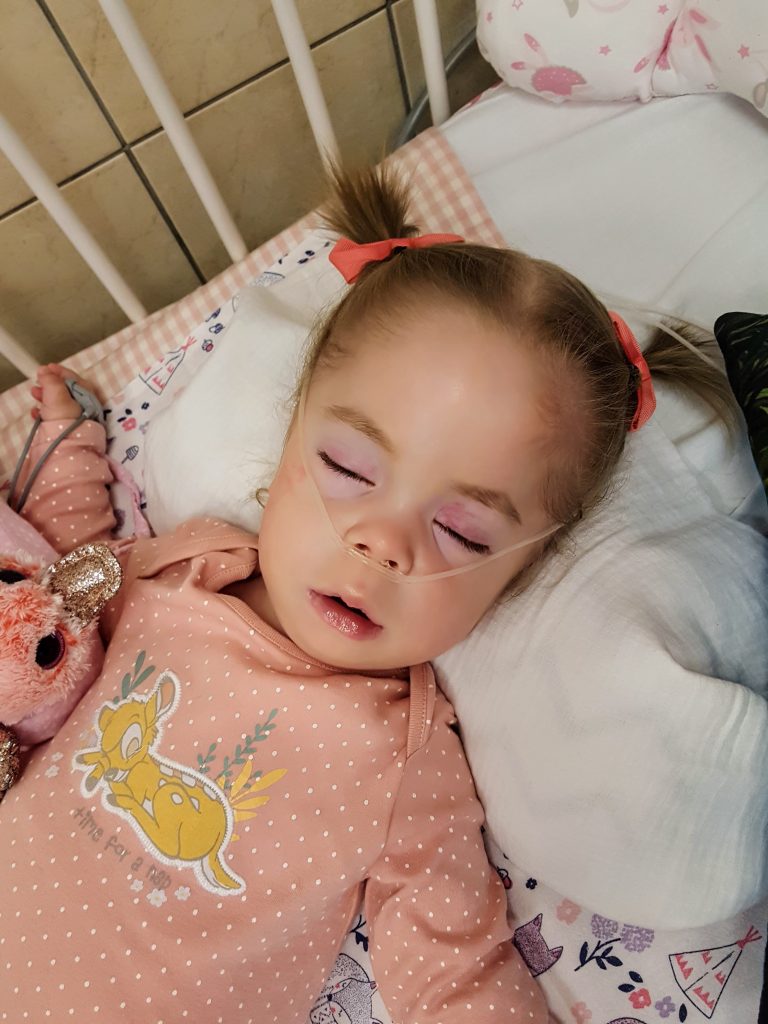
Mila was born on April 27, 2014 as a normal little girl in Mönchengladbach. She grew up normally and developed during the first three years of her life wonderfully. Nothing indicated the fatal disease. Shortly after her third birthday, Mila changed. This change became apparent in the form of speech difficulties and continued with increasing limitations in walking and movement. Her coordination skills also decreased day by day.
The parents initially consulted her pediatrician in Jüchen, but he was initially only able to treat the symptoms and referred Mila to a children’s rehab clinic in Meerbusch. The accompanying symptoms of the disease could be improved there by therapies. Unfortunately, the symptomatic medication against her numerous seizures did not help the young girl.
The diagnosis
On March 28, 2019, the moment of truth had arrived. Mila was given her definitive diagnosis at the University Hospital in Düsseldorf. Gangliosidosis 1 type 2, known as Derry syndrome. At the same time, it was her death sentence.


The decease
The GM1 Gangliosidosis is a very rare hereditary lysosomal storage disease that takes place in the cells. It is the deficiency of the enzyme beta-galactosidase. This enzyme is found in the cells of the nervous system, where it is responsible for the degradation and thus for the detoxification of three toxic substances, the so-called gangliosides.
In children, the clinical picture takes the form of organomegaly, an abnormal enlargement of the internal organs. Skeletal dysplasia, an impairment of bone and cartilage growth, as well as heart problems and numerous neurological impairments manifest themselves in a wide variety of forms.
This diagnosis changed Mila’s life and that of her parents tremendously. Yet giving up was not an option.
The father initially turned to the pharmacist Oliver Dienst, feeling helpless. Together they started to search and investigate this disease.
Distribution in the World
Of the more than 6,000 Gangliosidosis (GM1) patients living in the world, the largest number is found in India with 2,740 and in Brazil with about 2,030 children, while in Europe about 500 children are affected by the rare disease. The number of unreported cases may be several times higher.
Race against Time
Within a very short time, the father and the pharmacist established a worldwide network, and by chance they came across the biochemist Dr. Axel Heinemann. The latter researched medical databases and thus intensified the search.
He found Japanese research reports from the 1980s on a substance that was associated with Gaucher’s disease. Like GM1 Gangliosidosis, Gaucher’s disease is caused by a lysosomal storage disorder. Also in this case, a genetically determined malfunction prevents the degradation of equally toxic substances.


The active Ingredient
Aloxistatin is the name of this substance. It was patented in 2015 by a Swiss company and tested in various storage disorders. The most promising use was a treatment in 2018, which increased beta-galactoidase activity of a Ukrainian child. But unfortunately, the use of the drug came too late. The Ukrainian child died a short time later as a result of the insidious disease. Further investigation of the use of the substance in children suffering from GM1 Gangliosidosis was not continued.
It was only through the research of the father, the biochemist, and the pharmacist that the substance was rediscovered. Now there was a hopeful substance, but it was still far from being a drug. And the guidelines in Germany specify the administration of unapproved drugs: you cannot just give a child any substance that someone in the world once did research with and try it out. Above all, toxicity must be ruled out by many studies. And the question: where to get the substance and how to finance it?
The Off-Label Study
The pediatrician Dr. Christina Lampe from the University Hospital of Giessen, who had dealt with predominantly rare diseases and not only knew the disease “GM1 Gangliosidosis” but had also had experience with it in the past, offered herself as an advisory body for a possible therapy for Mila.
Mila’s therapy was to be approved by the health insurance company as part of an off-label study.
The Start of Therapy
At the beginning, the substance was still purchased from Estonia’s capital, Tallinn. 50 g at a price of 100,000 euros. And with the substance there was no drug yet. The question was, how should Mila take the substance? Swallow it? Drink it? As a suppository? Inhale? In our search for the most suitable galenic option, we decided on capsule production. And how much of the “expensive powder” should go into each capsule? What is the dose for a child? Neither the form of application, nor the dose were known. So, we tried and tested, relying on Mila’s help, and slowly increased the dosage.


A ray of hope
Mila was in very poor condition at the time of initial administration with loss of gait, severe motor problems, ataxia, seizures, loss of speech, and muscle hypotonia. After several weeks of administration of the substance, the activity of beta-galactosidase increased significantly in the presence of aloxistatin in fibroblasts, which are cells of connective tissue. Mila took 50mg 3 times a day with her meals from then on. The capsules were opened before use and the powder was given “pure”.
After 6 months of this “treatment” Mila is more alert, more concentrated and much fitter. Since then she has been smiling again from morning to evening. In particular, it is a huge relief for the parents that after taking aloxistatin, Mila clearly draws attention to the fact that she has to go to the toilet.
Feared unknown side effects did not materialize.
The second Kid: Biborkla
What seemed to be a miracle was now to be tested on another 18-month-old kid from Hungary. The Hungarian girl Biborkla was in a very serious condition with general edema at the time of the first administration. She was unable to move her head and limbs.
She suffered from seizures and was artificially ventilated with 0.5 – 1 liter of oxygen per minute. She suffered from cardiac, respiratory and digestive problems with excretory disorders.
Since May 2022, the young, critically ill patient has been treated with Aloxistatin and, like Mila, has shown improvement after a short time. The number of seizures reduced extremely, the edema reabsorbed completely, the oxygen supply was reduced to 0.25-0.5 liters per minute and the volume of her urine increased.
Her stomach decreased in size and she began to move her head and limbs on her own. Meanwhile, she no longer needs oxygen and can smile at her parents again. Again, there have been no unexpected side effects to date.
Project outline
Development of an aloxistatin-based oral delivery system using inclusion compounds
Now it is time to save the lives of many more children in this world.
This development project seeks to develop an aloxistatin-containing oral dosage form based on a formulation drug. To improve the absorption rate and resulting increased bioavailability, the formulation of an inclusion compound of aloxistatin with an adequate complexing agent is the focus of the joint project.
In particular, the following objectives are to be pursued:
• Identification of a suitable complexing agent for the preparation of a hydrophilic inclusion compound of aloxistatin
• Determination of the optimum substrate solubility in water
• Solubility testing by ultraviolet-visible spectroscopy (UV-Vis) and/or high-performance liquid chromatography (HPLC) and validation of the methods
• Qualitative characterization of the aloxistatin inclusion compound by FT-IR spectroscopy and differential scanning calorimetry (DSC) and, if necessary, by the application of other spectroscopic techniques (e.g., 1H NMR techniques)
• Galenic development of an oral dosage form
• Generation of stability data of the oral dosage form and total aerobic count (TAMC), if necessary preservative loading test
The father, Lothar Möller, has already collected just under 1 million euros. For investors, this is not only an affair of the heart, but perhaps also an economic success at some point. Because the market to be served is large. Even if 6,000 children are initially just a start, this beginning is worth every penny.
But one thing is certain. Time is against Mila and the other children.
Please add the message “Mila” to your PayPal donation
You can also donate by bank transfer
Recipient: Löwenkinder Viersen
Purpose of use: MILA
Account: Sparkasse Krefeld
IBAN : DE65 3205 0000 0000 4708 80
You will receive a donation receipt
Project procedure and concept
But this requires funds. The expected costs are about 250,000 €.
AP 1. Identification of a suitable complexing agent for the complexation of aloxistatin
- Preliminary tests with different complexing agents
- Identification of a suitable process for complexation
AP 2. development of a stable solution of the inclusion compound
- Development of a stable solution with the aloxistatin inclusion compound in a sufficient, therapeutically useful concentration
AP 3. solubility testing by spectroscopic methods.
- Preliminary testing to determine a suitable method for identifying and quantifying the aloxistatin inclusion compound using ultraviolet-visible spectroscopy (UV-Vis) and/or high-performance liquid chromatography (HPLC)
- Development and validation of the spectroscopic and/or chromatographic analytical procedure.
AP 4. Qualitative characterization of the aloxistatin inclusion compound
- Qualitative characterization of the aloxistatin inclusion compound by FT-IR spectroscopy and differential scanning calorimetry (DSC) as well as other spectroscopic methods (e.g. 1H-NMR spectroscopy), if necessary.
AP 5. Galenic development of the oral dosage form
- Galenic formulation development to a dosage form suitable for application
AP 6. method validation for stability testing
- Development and validation of a chromatographic and/or spectroscopic analytical method for stability testing of the oral formulation
AP 7. generation of stability data of the oral formulation
- Storage at different climatic zones (25°C and 40°C) and testing for stability and package compatibility
- Total Aerobic Microbial Count (TAMC) – at the beginning and end of the stability period.
- If applicable, performance of an upstream “test for sufficient preservation” in case of formulation of a liquid preparation